Articles from Sensydia

Sensydia, a clinical-stage non-invasive cardiac assessment company, announced today that University of Minnesota investigators presented positive clinical findings from a recent study evaluating the company’s AI-powered, non-invasive Cardiac Performance System (CPS™) in a poster presentation at the American College of Cardiology (ACC) 2025 Annual Scientific Session & Expo at the McCormick Place Convention Center in Chicago, IL.
By Sensydia · Via Business Wire · March 30, 2025
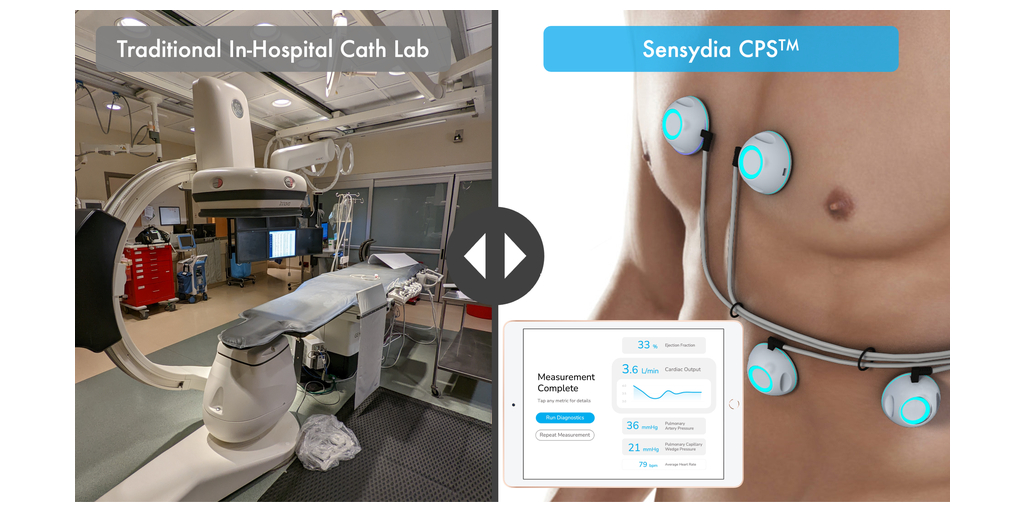
Non-invasive cardiac assessment company Sensydia announced today that it has completed its 50-subject development study at the University of Minnesota (UMN). This study was conducted at UMN to collect data for its innovative AI-powered, non-invasive Cardiac Performance System (CPS™) that uses heart sound analysis to enable earlier detection and more effective therapy guidance for patients suffering from heart failure and pulmonary hypertension.
By Sensydia · Via Business Wire · January 16, 2024

Non-invasive cardiac diagnostic company Sensydia announced today that it was awarded a Fast-Track Small Business grant from the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH).
By Sensydia · Via Business Wire · June 5, 2023

Non-invasive cardiac assessment company Sensydia today announced it has raised approximately $8M in new funding from an expanded syndicate of investors.
By Sensydia · Via Business Wire · May 9, 2023
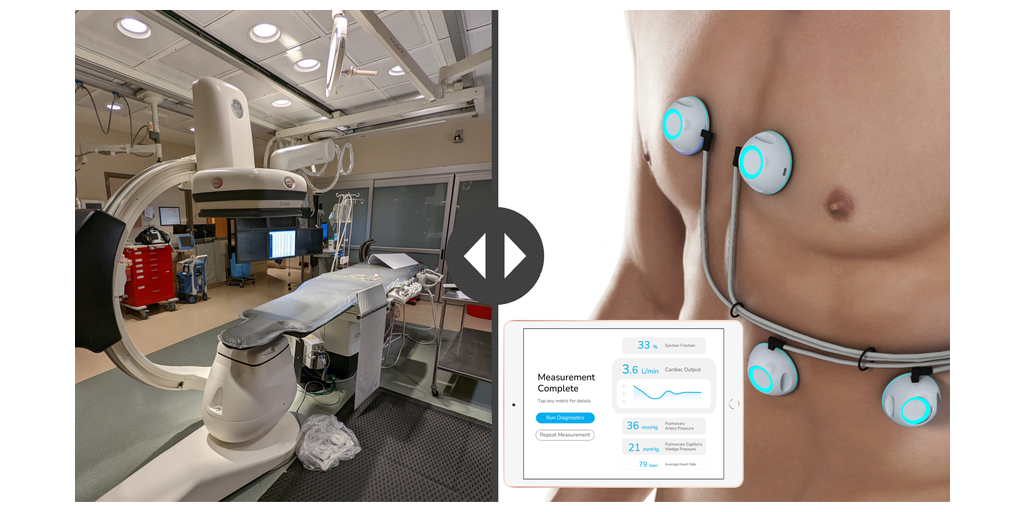
Non-invasive cardiac assessment company Sensydia announced today that it has completed its 225-subject development study at the University of Pittsburgh Medical Center (UPMC). This study was conducted at UPMC to collect data for its innovative AI-powered, non-invasive Cardiac Performance System (CPS™) that uses heart sound analysis to enable earlier detection and more effective therapy guidance for patients suffering from heart failure and pulmonary hypertension.
By Sensydia · Via Business Wire · February 6, 2023
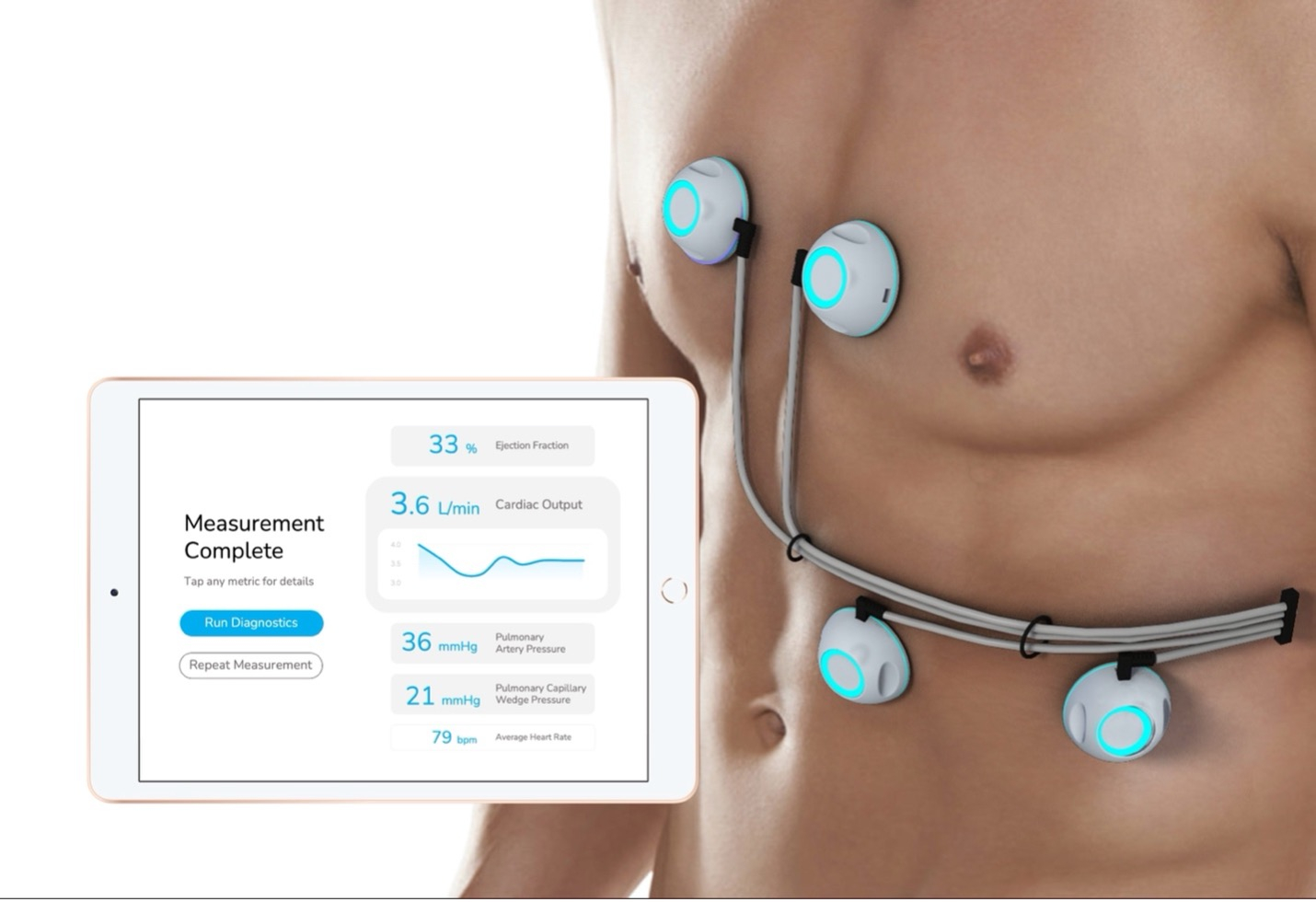
Sensydia, an innovator in rapid, non-invasive measurement of critical cardiac function, today announced that its Cardiac Performance System (CPSTM) has been granted Breakthrough Device Designation by the United States Food and Drug Administration (FDA). As part of this Breakthrough Device Designation, the FDA will work closely with Sensydia to expedite the development of CPS and prioritize the review of subsequent regulatory submissions.
By Sensydia · Via Business Wire · January 19, 2022
